Highly diastereoselective C → N acyl rearrangement in polysubstituted pyrrolidine 2,2-dicarboxylates. Stereocontrolled synthesis of densely functionalized prolines†
Abstract
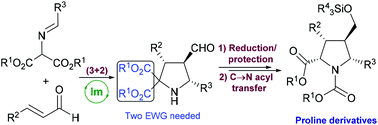
An efficient and easy protocol for promoting a C → N acyl transfer reaction in diverse enantiopure densely substituted pyrrolidine 2,2-dicarboxylates was performed, yielding the corresponding proline ester derivatives with yields in the range of 68–99% and total diastereoselection (dr > 95 : 5 in all cases). The substitution patterns both at C-3 and C-5 present in starting pyrrolidines as well as the viability of presenting different alkoxycarbonyl moieties have been evaluated observing good reaction performance in all cases.


 Please wait while we load your content...
Please wait while we load your content...