Influence of halogen substitution on aggregation-induced near infrared emission of borondifluoride complexes of 2′-hydroxychalcones†
Abstract
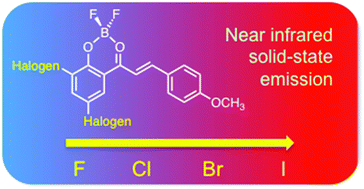
The generation of organic dyes displaying intense red and near infrared fluorescence emission in solution and in the solid state is a topic of intense current interest. In this study, we present the synthesis and investigation of nine new derivatives of borondifluoride complexes of 2′-hydroxychalcones: four homo- and five hetero-substituted compounds X–X and X–Y (X, Y = F, Cl, Br, and I), where two halogen atoms are attached ortho and para (X and Y respectively in X–Y) to the 2′-hydroxyl group. UV-vis absorption fluorescence spectra in solution and in the solid state, DFT calculations, and analyses of single-crystal structures were performed. For all compounds, a fluorescence emission enhancement was clearly observed when passing from the solution (DCM) to the crystal (up to more than 10 fold). These molecules thus exhibit aggregation-induced enhanced emission (AIEE) with fluorescence maxima ranging from 654 nm (F–F) to 807 nm (I–I) in the solid state. The highest fluorescence quantum yield value within the series is reached for Cl–Cl (24% at 730 nm) whilst I–Br shows a rather good efficiency (2.5% at 806 nm) despite the presence of the two heavy iodine and bromine atoms. This study sheds light on the role of the halogen substitution on crystalline packing and solid-state emission properties of the chalcone–BF2 dyes. We outline that using halogen atoms provides a useful cocktail of steric, electrostatic and photophysical ingredients upon which to base the generation of near infrared emitting organic solids.
- This article is part of the themed collection: Recent Progress on Aggregation-Induced Emission


 Please wait while we load your content...
Please wait while we load your content...