Hydrogels based on pH-responsive reversible carbon–nitrogen double-bond linkages for biomedical applications
Abstract
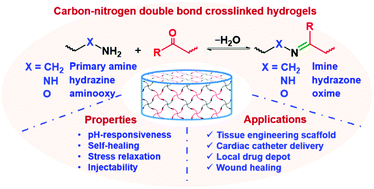
Covalently-crosslinked hydrogels are formed due to the generation of new covalent crosslinking bonds in situ, leading to the formation of covalently-linked networks. However, traditional polymerization might introduce potential toxicity issues. Recently, carbonyl-condensation reactions that form imine, hydrazone or oxime bonds have become important tools for the preparation of covalently-crosslinked hydrogels for biomedical applications. The formation of imine, hydrazone or oxime linkages occurs under mild conditions with water as the only byproduct. The reactions are reversible and pH-responsive, and are dependent on the chemical structure of the substrates. In this review, we focus on the utilization of the condensation reactions between nucleophiles and carbonyl groups as crosslinking strategies to prepare hydrogels with physiochemical properties sensitive to biologically relevant stimuli. A brief account of the formation mechanisms and stabilities of these linker moieties will help to understand how reactant structure controls the gelation and degradation of hydrogels. Natural and synthetic polymers that are commonly employed as building blocks and synthetic methods that are selected for decoration of different polymers with respective functional groups will be summarized. Then, the preparation and biomedical applications of the hydrogels based on imine/hydrazone/oxime formation will be presented respectively, with special focus on the developments in the past decade. This review will provide insight into the design of novel biocompatible, stimuli-responsive hydrogels based on dynamic linkages for various biomedical applications.
- This article is part of the themed collections: Stimuli-responsive materials and 2018 Materials Chemistry Frontiers Review-type Articles


 Please wait while we load your content...
Please wait while we load your content...