Ratiometric real-time monitoring of hydroxyapatite–doxorubicin nanotheranostic agents for on-demand tumor targeted chemotherapy†
Abstract
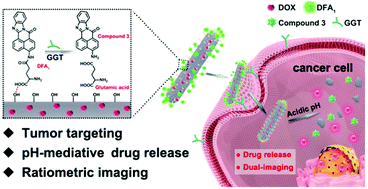
We reported dual-fluorescent hydroxyapatite–doxorubicin (DOX) (DDHAP) nanocomposites for tumor-targeted therapy. A newly designed fluorescent tumor-targeting group, DFA1, is grafted onto the nanoparticle surface, which can enhance the cellular uptake of DDHAP by binding to γ-glutamyl transpeptidase (GGT), a cell surface-associated enzyme that is overexpressed on cancer cell membranes. This DFA1 moiety could undergo fluorescence quenching after binding to GGT, and the whole nanocomposite collapsed under the cancerous pH condition, thereby releasing the free fluorescent DOX as an effective anticancer drug. Thus, ratiometric fluorescence tracking can be built up by measuring the DOX/DFA1 fluorescence ratio. The dual fluorescence for ratiometric real-time tracking of the nanotherapeutic agents provides a new platform for better understanding the detailed process of their cellular uptake and intracellular dissociation. Moreover, as confirmed by in vivo studies, hydroxyapatite–DOX nanotheranostic agents demonstrate specific tumor-targeting, efficient tumor tissue penetrating and excellent tumor inhibiting effects. Nanotheranostic agents based on DDHAP show high potential for effective cancer treatment in future clinical settings.


 Please wait while we load your content...
Please wait while we load your content...