Controlling the canonical/zwitterionic balance through intramolecular proton transfer: a strategy for vapochromism†
Abstract
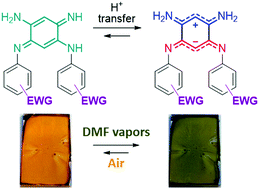
A series of N-substituted diamino-benzoquinone diimines featuring tunable aryl electron-withdrawing functions were synthesized. For the first time, the subtle variation of electronic richness allows controlling the canonical vs. zwitterionic equilibrium through the tuning of an intramolecular proton transfer. The establishment of a ground-state zwitterionic electronic structure, in lieu of the canonical one, within a monocyclic architecture is evidenced by the X-ray structure of one compound (4) and the telltale absorption spectra of the whole series that display an unexpected signature at low energy (ca. 700 nm). The evolution of this peculiar band is concomitant with the electron-withdrawing strength of the aryl substituents and theoretical calculations highlight that this band can be attributed to a charge transfer transition from the anionic cyanine towards the cationic cyanine. Finally, such a color change is advantageously used for the rapid and naked-eye detection of N,N-dimethylformamide using 3a as a vapochromic material. This latter experiment shows the first case of intramolecular solid-state proton transfer occurring in a polar environment.


 Please wait while we load your content...
Please wait while we load your content...