Synthesis of porous nitrogen doped zinc oxide nanostructures using a novel paper mediated template method and their photocatalytic study for dye degradation under natural sunlight†
Abstract
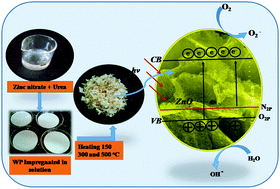
N-Doped zinc oxide (N-ZnO) nanostructures were prepared using a novel paper mediated template combustion method. The precursors were impregnated on filter papers and thermally processed to obtain porous N-ZnO nanostructures. The XRD patterns of the as-synthesized N-ZnO confirm the formation of the wurtzite phase, and the broad diffraction peaks confirm the nanocrystalline nature of the product. The TEM and SEM images show spherical shaped 20–30 nm N-ZnO nanoparticles. The UV-visible absorbance spectra of the product show a red shift in the spectra with increasing doping concentration of nitrogen in ZnO. This red shift was due to incorporation of nitrogen into the ZnO lattice. Photoluminescence spectra indicate a strong emission peak at 391 nm which corresponds to band gap emission. Considering the band gap of the as-synthesized N-ZnO to be well within the visible region, the photocatalytic activity for the degradation of methylene blue (MB) and rhodamine (RhB) dyes was studied under direct sunlight using the as-synthesized N-ZnO. The N-ZnO prepared with a higher amount of urea (1 : 8) shows the highest photocatalytic activity as compared to the other synthesized N-ZnO samples towards the degradation of MB and RhB, i.e. complete degradation of MB in 30 min and RhB in 45 min. Our novel synthesis approach provides uniformly distributed nanosized spherical particles having higher photocatalytic activity as compared to other reported methods.


 Please wait while we load your content...
Please wait while we load your content...