A novel co-crystallization molecular ferroelectric induced by the ordering of sulphate anions and hydrogen atoms†
Abstract
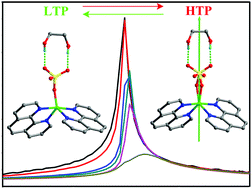
Molecular ferroelectrics based on co-crystallization complexes have been very seldom reported before. Here we present the interesting case of the co-crystallization copper complex [EG]·[Cu(phen)2·SO4], 1 (EG = ethylene glycol, phen = 1,10-phenanthroline), which displays a reversible paraelectric–ferroelectric phase transition at 272 K. 1 undergoes symmetry breaking from C2/c to Cc at around 272 K which is triggered by the disorder–order transition of sulphate anions and hydrogen bonds. The real component of the dielectric constant versus temperature shows very large maxima for dielectric anomalies, corresponding to the phase transition. The activation energy is about 84.2 kJ mol−1, which can be calculated using the Arrhenius equation τ = τ0 exp(Ea/kBT) in a graph of dielectric loss changes with temperature at different frequencies. The measurement of ferroelectric properties shows a maximum spontaneous polarization (Ps) of ca. 3.18 μC cm−2 with a coercive field (Ec) of 1.2 kV cm−1. The phase transition sequence of 1 was further verified by differential scanning calorimetry (DSC) with one heat anomaly peak observed at around 272 K.
- This article is part of the themed collection: Inorganic Chemistry Frontiers HOT articles for 2018


 Please wait while we load your content...
Please wait while we load your content...