Tailorable degradation of pH-responsive all polyether micelles via copolymerisation with varying acetal groups†
Abstract
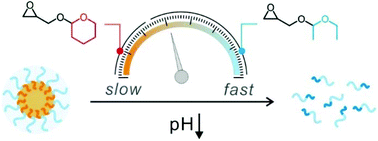
Smart drug delivery in a site-specific and time-controlled manner is critical for reducing the side effects of the drug while maximizing the therapeutic efficacy. Herein, we describe an efficient approach to control the degradation kinetics of polyether micelles under acidic conditions using random copolymers of functional epoxide monomers bearing different acetal groups. The amphiphilic block copolymers, poly(ethylene glycol)-block-poly(ethoxyethyl glycidyl ether-co-tetrahydropyranyl glycidyl ether)s (PEG-b-P(EEGE-co-TGE))s, are synthesized by the anionic ring-opening polymerisation of the pH-responsive novel epoxide monomers ethoxyethyl glycidyl ether (EEGE) and tetrahydropyranyl glycidyl ether (TGE) in varying ratios. The random block copolymers are carefully characterized by 1H NMR, GPC, and DSC and the copolymerisation kinetics are evaluated using in situ1H NMR analysis. The critical micelle concentrations, loading efficiencies, and size distributions of the copolymer micelles show a saturation point over a critical TGE ratio. Interestingly, the degradation and subsequent release kinetics of the micelles under acidic conditions are remarkably different when the composition of the acetal groups is varied. The superior biocompatibility coupled with the highly tailorable release kinetics is anticipated to lead to a versatile platform for smart drug delivery systems.


 Please wait while we load your content...
Please wait while we load your content...