Helicity control of π-conjugated foldamers containing d-glucose-based single enantiomeric units as a chiral source†
Abstract
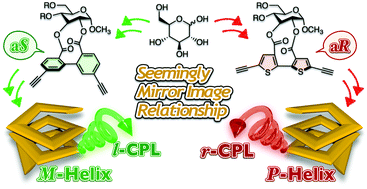
An optically active bithiophene derivative containing a D-glucose residue and two ethynyl groups ((aR)-7) was synthesized and copolymerized with 2,5-diiodothiophene through Sonogashira–Hagihara cross-coupling. The absorption, circular dichroism, photoluminescence and circularly polarized luminescence properties of the resulting polymer (poly-7) were investigated under various solution conditions. Poly-7 exhibited a clear solvent dependence of the optical and chiroptical properties as a result of the interconversion between random-coil and right-handed helical backbones. We also demonstrated that poly-7 emitted right-handed circularly polarized light upon ultraviolet irradiation in the helically folded state. Based on the contrasting results for a previously reported polymer (poly-A), which contained biphenyl units instead of bithiophene units in the main chain, it can be concluded that seemingly mirror-imaged chiral materials can be prepared using a single enantiomer of glucose without the need for the unnatural L-isomer.


 Please wait while we load your content...
Please wait while we load your content...