Exquisite regulation of supramolecular equilibrium polymers in water: chain stoppers control length, polydispersity and viscoelasticity†
Abstract
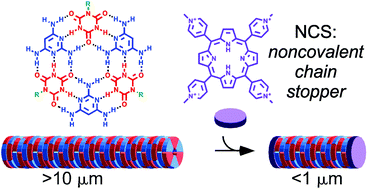
Supramolecular polymers are increasingly important materials for applications that require the controlled assembly and disassembly of structures having well-defined shapes and properties. Subtle changes in molecular structure and solvent properties can profoundly affect these materials because of the relatively weak noncovalent interactions that govern their formation. We report the discovery that positively charged small molecules that are planar, or have planar elements, can be employed as noncovalent chain stoppers (NCSs) to control the length of supramolecular polymers in aqueous solution. The supramolecular polymers are composed of 2,4,6-triaminopyrimidine and a modified cyanuric acid, monomers that assemble through interactions that mimic the base pairs of DNA. These assemblies are equilibrium polymers, a type of supramolecular polymers that, in the absence of any chain stoppers, are extremely long (e.g., multiple microns in length). The lengths of these assemblies are controlled by a NCS in a concentration-dependent and compound-specific manner, giving rise to supramolecular polymers with low polydispersity. This behavior is attributed to the homogeneity of the NCS distribution within the system, and their “ideal-gas-like” number density fluctuations. These supramolecular polymers form physical hydrogel with viscoelastic properties that can be tuned by the addition of a NCS, thereby demonstrating the potential of this approach for controlling the dimensions of supramolecular polymers in water as well as the macroscopic properties of materials formed by such polymers.


 Please wait while we load your content...
Please wait while we load your content...