An efficient lactone-to-lactam conversion for the synthesis of thiophene Pechmann lactam and the characterization of polymers thereof†
Abstract
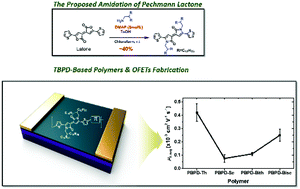
Despite having unique structural features, e.g., high co-planarity and a strong polar bicyclic lactam structure, thiophene bipyrrolylidene-2,2′(1H,1′H)-dione (TBPD) has been less explored as a dye, mainly due to the quite low yield in its synthesis via lactone-to-lactam conversion. We reported an efficient methodology for synthesizing TBPD in high yield using p-toluenesulfonic acid monohydrate and a catalytic amount of 4-dimethylaminopyridine in the chloroform solvent. From the newly synthesized series of TBPD-based donor–acceptor-type polymers, we fabricated organic field-effect transistors (OFETs), which were subjected to a systematic study on the relationship between film microstructure and charge transport. Among them, the annealed PTBPD-Th film revealed a more ordered lamellar packing with the highest number of interlayers and preferential edge-on orientation, yielding the best hole mobility (up to 0.46 cm2 V−1 s−1). The improved synthesis of TBPD and our findings concerning related polymers could promote further research and development associated with the TBPD unit.


 Please wait while we load your content...
Please wait while we load your content...