Copolymerization of ethylene with styrene catalyzed by a scandium catalyst†
Abstract
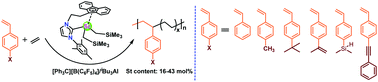
Copolymerization of ethylene (E) and styrene (St) was successfully achieved by using a scandium catalyst to produce a series of pseudo-random copolymers. The incorporation content of the St monomer could be tuned from 16.1 mol% to 43.2 mol% swiftly through varying the St to E feeding mole ratio and polymerization time. The highest St content in the copolymer is lower than 50 mol% even at a feeding mole ratio as high as St : E = 31 : 1 due to the steric hindrance around the active metal center. This is consistent with the monomer reactivity ratios of rE = 14.7 and rst = 0.0036 calculated according to the Fineman–Ross equation. This catalytic performance was extended to other styrene derivatives, such as 4-methylstyrene, 4-tert-butylstyrene, 4-isopropenestyrene, 4-vinylphenyl dimethylsilane and 4-phenylethynylstyrene. The resulting E–St copolymers have no consecutive St sequences and show only one glass transition temperature varying from −21.2 °C to 18.2 °C with the St content. DFT simulation reveals the copolymerization mechanism.


 Please wait while we load your content...
Please wait while we load your content...