Dynamic diselenide-containing polyesters from alcoholysis/oxidation of γ-butyroselenolactone†
Abstract
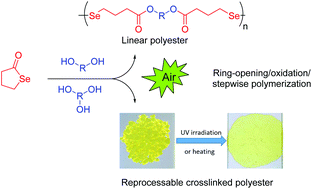
A versatile protocol for the synthesis of a variety of multiresponsive diselenide-containing polyesters was investigated. It consists of a one-pot, two-step process with the generation of a selenol by nucleophilic ring opening of γ-butyroselenolactone with a broad range of alcohols in situ, followed by the transformation of the obtained compounds to the corresponding diselenides through a spontaneous oxidative coupling reaction. First, the influence of the catalyst choice, alcohol structure, and solvents on the reaction efficiency was examined. After elaboration of this one-pot reaction, a number of routes, all starting from γ-butyroselenolactone, have been developed for the successful synthesis of linear and crosslinked diselenide-containing polyesters via a mild, straightforward process. The structure of diselenide compounds and polymers was carefully characterized by 1H, 13C, 77Se NMR, FT-IR, HRMS, and SEC. In addition, it has been found that the diselenide-containing polyester networks showed fast reprocessing at a mild temperature (70 °C) or by making use of UV irradiation.


 Please wait while we load your content...
Please wait while we load your content...