Design and synthesis of an AIE-active polymeric H2S-donor with capacity for self-tracking†
Abstract
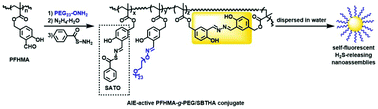
The design and synthesis of a new type of self-fluorescent polymeric H2S-donor was reported for the first time. The reversible addition–fragmentation chain transfer (RAFT) polymerization of 3-formyl-4-hydroxybenzyl methacrylate (FHMA) afforded a structurally well-defined polymer poly(FHMA) (PFHMA) containing reactive salicylaldehyde groups that can serve as chemical handles for subsequent postpolymerization functionalization. Some of the aldehyde groups in PFHMA were then reacted sequentially with aminooxy-terminated PEG (PEG-ONH2) and hydrazine to incorporate water-soluble and biocompatible PEG side chains and salicylaldazine AIE fluorogens into PFHMA, affording an AIE-active PFHMA-g-PEG graft copolymer. The remaining salicylaldehyde units in the AIE-active PFHMA-g-PEG were utilized for the conjugation of S-benzoylthiohydroxylamine (SBTHA) to yield an AIE-active PFHMA-g-PEG/SBTHA conjugate that contains S-aroylthiooxime (SATO) units. Both AIE-active PFHMA-g-PEG and PFHMA-g-PEG/SBTHA conjugates showed no obvious cytotoxicity (cell viability >85%) even at high concentrations of up to 100 μg·mL−1, demonstrating good biocompatibilities. Triggered with cysteine or glutathione (1 mM), PFHMA-g-PEG/SBTHA conjugates ([SATO moieties] = 100 μM) could release H2S slowly with a ∼55 min peak time or ∼70 min peak time respectively. Thanks to the water-soluble PEG side chains, the resulting self-fluorescent H2S-releasing polymer could be dispersed in water, and its AIE attribute allowed for the visual representation within living cells via fluorescence imaging.


 Please wait while we load your content...
Please wait while we load your content...