The effect of linker length on ConA and DC-SIGN binding of S-glucosyl functionalized poly(2-oxazoline)s†
Abstract
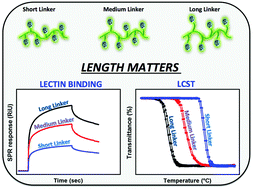
A new monomer, 2-[2-((2,3,4,6-tetra-O-acetyl-β-D-glucopyranosylthio)propyl)]-2-oxazoline (Ac4Glc-S-Ox), was synthesized by direct addition of 2,3,4,6-tetra-O-acetyl-1-thio-β-D-glucopyranose (Ac4Glc-SH) to 2-isopropenyl-2-oxazoline (iPOx) in the presence of solid butyl amine resin via a thiol–ene click reaction. The living cationic ring-opening polymerization was performed to prepare copolymers of Ac4Glc-S-Ox with 2-ethyl-2-oxazoline (EtOx). In order to systematically investigate the effect of the S-glucosyl substituent linked to the polymer backbone with different linkers on the cloud point and the binding ability, another series of glycopolymers was prepared by a post polymerization modification method. Copolymers of 2-decenyl-2-oxazoline and 2-butenyl-2-oxazoline with EtOx were first polymerized and then reacted with Ac4Glc-SH. The obtained glycopolymers exhibited lower critical solution temperature behavior that could be tuned easily by manipulating the alkyl linker length. Moreover, the binding results obtained using both turbidimetry and surface plasmon resonance techniques suggested that the relationship of the linker with the polymer backbone has a critical influence on glycopolymer–lectin binding behaviour.


 Please wait while we load your content...
Please wait while we load your content...