A thermo-responsive polyurethane organogel for norfloxacin delivery†
Abstract
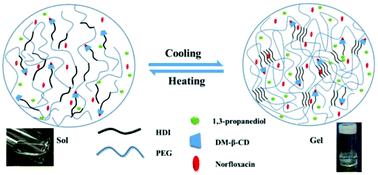
To overcome the constraints presented by the conventional hydrogel-based skin patch, including poor transdermal penetration activity and reluctant release of the encapsulated lipophilic drugs, a facile organogel constituted of amphiphilic hexamethylene diisocyanate (HDI)-based polyurethanes (PUs) was developed in 1,3-propanediol, and the amphiphilic components of poly (ethylene glycol) (PEG) and HDI allow for the formulation of distinctive segregated domains of PEG and HDI based on the inter- and intra-molecular interactions between HDI segments. Of note, a convenient cooling down process allows for the formulation of a stable and homogenous organogel. In addition, a functional host 2,6-dimethyl-β-cyclodextrin (CD) group, acting as a drug reservoir, was included in PEG-HDI, characterized by its gel to sol state transition in the range of 24–36 °C, for the encapsulation of a pharmaceutical compound (norfloxacin), consequently contributing to a minimal drug release profile at 4 °C but an accelerated rate of drug release at physiological temperature. The in vitro drug release and transdermal experimental results show that the organogel as a drug carrier was capable of releasing the drug efficiently, improving the transdermal delivery, and exhibiting bactericidal activity over 80% in vivo. Moreover, the introduced CD moieties could increase the solubility of lipophilic drugs and the dissociation rate in biological milieu, which appears to benefit the promoted drug release rate and transdermal efficiency as compared to a CD-free organogel.


 Please wait while we load your content...
Please wait while we load your content...