Organocatalytic enantioselective synthesis of acyclic pyrimidine nucleosides by aza-Michael reaction†
Abstract
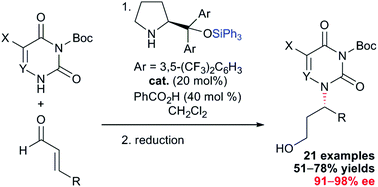
An efficient diarylprolinol triphenylsilyl ether-catalyzed enantioselective aza-Michael reaction of pyrimidines as N-centered nucleophiles to α,β-unsaturated aldehydes, followed by reduction, provided chiral acyclic pyrimidine nucleosides in good yields (51–78% yields for two steps) and excellent enantioselectivities (91–98% ee). In addition, the chiral acyclic pyrimidine nucleoside having the tert-butyldiphenylsilyl-protected hydroxyl substituent was successfully applied to the synthesis of the corresponding chiral cyclic pyrimidine nucleoside analogue bearing the tetrahydrofuranyl ring.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...