Superelectrophilic activation of 1-nitronaphthalene in the presence of aluminum chloride. Reactions with benzene and cyclohexane†
Abstract
1-Nitronaphthalene smoothly reacts with benzene and undergoes selective reduction with cyclohexane in the presence of aluminum chloride to give 2,4,4-triphenyl-3,4-dihydronaphthalen-1(2H)-one oxime and 5,6,7,8-tetrahydro-1-naphthylamine, respectively. The mechanistic aspects of these and related reactions are discussed on the basis of DFT, providing insight into the protonation behavior of 1-nitronaphthalene coordinated to AlCl3.
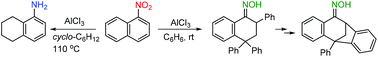
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...