Syntheses and in vitro biological evaluation of S1PR1 ligands and PET studies of four F-18 labeled radiotracers in the brain of nonhuman primates†
Abstract
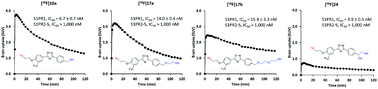
A series of seventeen hydroxyl-containing sphingosine 1-phosphate receptor 1 (S1PR1) ligands were designed and synthesized. Their in vitro binding potencies were determined using [32P]S1P competitive binding assays. Compounds 10a, 17a, 17b, and 24 exhibited high S1PR1 binding potencies with IC50 values ranging from 3.9 to 15.4 nM and also displayed high selectivity for S1PR1 over other S1P receptor subtypes (IC50 > 1000 nM for S1PR2–5). The most potent compounds 10a, 17a, 17b, and 24 were subsequently radiolabeled with F-18 in high yields and purities. MicroPET studies in cynomolgus macaque showed that [18F]10a, [18F]17a, and [18F]17b but not [18F]24 crossed the blood brain barrier and had high initial brain uptake. Further validation of [18F]10a, [18F]17a, and [18F]17b in preclinical models of neuroinflammation is warranted to identify a suitable PET radioligand to quantify S1PR1 expression in vivo as a metric of an inflammatory response.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...