Nazarov cyclisations initiated by DDQ-oxidised pentadienyl ether: a mechanistic investigation from the DFT perspective†
Abstract
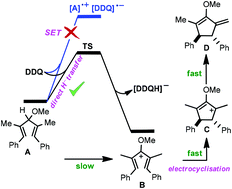
The Nazarov cyclisation is an important and reliable reaction for the synthesis of cyclopentenones. Density functional theory (DFT) has been utilised to study the mechanism of Nazarov cyclisations initiated by oxidation of pentadienyl ethers by a benzoquinone derivative (DDQ), as recently reported by West et al. (Angew. Chem., Int. Ed., 2017, 56, 6335). We determined that the reaction is most likely initiated by a hydride transfer from the pentadienyl ether to an oxygen of DDQ through a concerted pathway and not a single electron transfer mechanism. This oxidation by hydride abstraction leads to the formation of a pentadienyl cation from which the 4π electrocyclisation occurs, giving an alkoxycyclopentenyl cation. The ensuing cation is subsequently deprotonated by the reduced DDQ to afford an enol ether product. Consistent with experimental results, the hydride transfer is calculated to be the rate determining step and it can be accelerated by using electron donating substituents on the pentadienyl ether substrate. Indeed, the electron donating substituents increase the HOMO energy of the ether, making it more reactive toward oxidation. It is predicted that an unsubstituted benzoquinone, due to having a higher lying LUMO, shows much less reactivity than DDQ. Interestingly, we found an excellent correlation between the hydride transfer activation energy and the gap between the ether HOMO and the benzoquinone LUMO. From this correlation, we propose a predictive formula for reactivity of different types of substrates in the corresponding reaction.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...