Efficient synthesis of esters through oxone-catalyzed dehydrogenation of carboxylic acids and alcohols†
Abstract
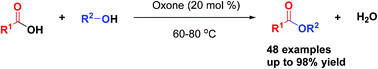
Since esters are important organic synthesis intermediates, an environmentally friendly oxone catalyzed-esterification of carboxylic acids with alcohols has been developed. A series of carboxylic acid esters are obtained in high yield. This strategy requires mild reaction conditions, providing an attractive alternative for the construction of valuable carbonyl esters. Electron-rich and electron-deficient groups are compatible with the standard conditions and a variety of substrates are demonstrated. Moreover, the reaction could easily be adapted to typical prodrugs, drugs and gram-scale synthesis.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...