Stapling of two PEGylated side chains increases the conformational stability of the WW domain via an entropic effect†
Abstract
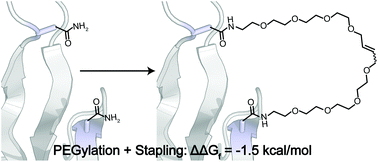
Hydrocarbon stapling and PEGylation are distinct strategies for enhancing the conformational stability and/or pharmacokinetic properties of peptide and protein drugs. Here we combine these approaches by incorporating asparagine-linked O-allyl PEG oligomers at two positions within the β-sheet protein WW, followed by stapling of the PEGs via olefin metathesis. The impact of stapling two sites that are close in primary sequence is small relative to the impact of PEGylation alone and depends strongly on PEG length. In contrast, stapling of two PEGs that are far apart in primary sequence but close in tertiary structure provides substantially more stabilization, derived mostly from an entropic effect. Comparison of PEGylation + stapling vs. alkylation + stapling at the same positions in WW reveals that both approaches provide similar overall levels of conformational stability.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...