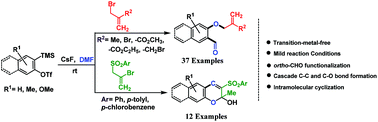
A cascade process for the synthesis of ortho-formyl allyl aryl ethers and 2H-chromen-2-ol derivatives from arynes via trapping of o-quinone methide with an activated alkene†
Abstract
A transition-metal free synthetic strategy has been developed for the direct synthesis of ortho-formyl substituted allyl aryl ethers via a cascade three-component coupling of arynes, activated alkene and N,N-dimethylformamide. The reaction proceeds via C–O and C–C bond cleavage as well as C–C and two new C–O bond formations in a single reaction vessel. The methodology provides a good yield of ortho-formyl substituted allyl aryl ethers. Moreover, the synthetic strategy has been utilized for the one-pot synthesis of 2H-chromen-2-ol derivatives.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...