Compatibility between the cysteine-cyclopentenedione reaction and the copper(i)-catalyzed azide–alkyne cycloaddition†
Abstract
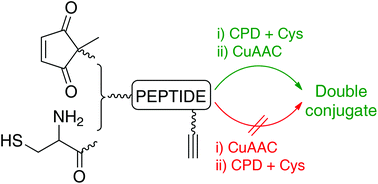
The cysteine-cyclopentenedione reaction can be combined with the copper(I)-catalyzed azide–alkyne cycloaddition provided that the former is carried out first. If not, the azide and the cyclopentenedione undergo a 1,3-dipolar cycloaddition, which furnishes triazole-containing compounds and products resulting from nitrogen loss. Both of these products were fully characterized. Attempts to obtain either of them as the main compound or to drive the reaction nearly to completion were unsuccessful, which points to the azide-cyclopentenedione reaction as not being useful for bioconjugation.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...