Tunable synthesis of quinolinone-fused isoquinolines through sequential one-pot nucleophilic addition and palladium-catalyzed intramolecular C–H alkenylation†
Abstract
An efficient sequential one-pot synthesis of fused heterocycles based on 4-quinolinone and isoquinoline scaffolds of biological interest has been developed. In all cases, the first nucleophilic addition of 2-aryl quinolin-4(1H)-ones to alkynyl bromides in tert-pentyl alcohol can proceed in a chemo-, regio- and stereoselective manner to give (Z)-N-(1-bromo-1-alken-2-yl)quinolin-4-ones at 110 °C. Sequentially, these in situ functionalized adducts can undergo direct intramolecular aromatic C–H alkenylation in the presence of PdCl2 (5 mol%) in mixed solvents (tert-pentyl alcohol/DMF = 1 : 1), affording the novel quinolinone-fused isoquinoline derivatives in good yields.
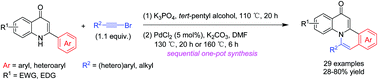
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...