Aerobic oxidation of 5-hydroxymethylfurfural to 5-hydroxymethyl-2-furancarboxylic acid and its derivatives by heterogeneous NHC-catalysis†
Abstract
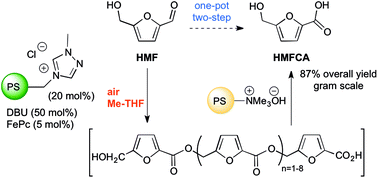
The application of the oxidative system composed of a heterogeneous triazolium pre-catalyst, iron(II) phthalocyanine and air is described for the selective conversion of 5-hydroxymethylfurfural (HMF) into the added-value 5-hydroxymethyl-2-furancarboxylic acid (HMFCA). The disclosed one-pot two-step procedure involved sequential oxidative esterifications of HMF to afford a polyester oligomer having hydroxyl and carboxyl terminal groups (Mw = 389–1258), which in turn was hydrolyzed by a supported base (Ambersep 900 OH) to yield HMFCA in 87% overall yield. The same strategy was adopted for the effective synthesis of ester and amide derivatives of HMFCA by nucleophilic depolymerization of the oligomeric intermediate with methanol and butylamine, respectively. The utilization of the disclosed oxidative system for the direct conversion of HMF and furfural into their corresponding ester, amide, and thioester derivatives is also reported.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...