Chemoselective synthesis of m-teraryls through ring transformation of 2H-pyran-2-ones by 2-(1-arylethylidene)-malononitriles†
Abstract
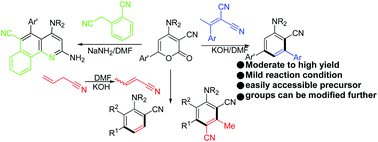
We have developed a simple, efficient and chemoselective approach for the synthesis of m-teraryls by the reaction of 6-aryl-2-oxo-4-(sec.amino)-2H-pyran-3-carbonitriles and 2-(1-arylethylidene)malononitriles under basic conditions. We used 6-aryl-2-oxo-4-methylsulfanyl-2H-pyran-3-carbonitriles as precursors and successfully afforded 5′-methylsulfanyl-[1,1′;3′,1′′]teraryl-4′-carbonitriles. We tried to understand the difference in the reactivity of structurally symmetrical molecules, such as allyl cyanide, 2-cyanomethylbenzonitrile and 2-(1-arylethylidene)malononitrile. The structure of 4′′-methyl-5′-(piperidin-1-yl)-[1,1′:3′,1′′-terphenyl]-4′-carbonitrile was confirmed by single crystal X-ray diffraction analysis.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...