Design of C2-symmetric alkaloidal chiral amphiphiles and configurational effects on self-assembly†
Abstract
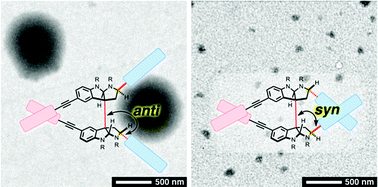
Alkaloids are a cornerstone in the development of medicinal and synthetic compounds due to their capability of specific recognition of targeted biomacromolecules, and uses in optical resolution and asymmetric reactions. To explore the untapped potential of the rigid and densely functionalized structures of alkaloids with precisely regulated configurations as optically active core scaffolds of self-assembling molecules, here we report the design, syntheses, chiroptical properties and self-assemblies of C2-symmetric alkaloidal amphiphiles with anti/syn stereochemical variations. Bispyrrolidinoindoline (BPI) was chosen as the optically active core scaffold. It was synthetically modified with hydrophobic alkyl chains and hydrophilic tetraethylene glycol tails to provide amphiphilicity. The anti/syn configurational differences in the amphiphiles significantly influenced the chiroptical, dynamic and supramolecular properties. Amphiphiles with anti-configurations responded to a solvent polarity change by altering their conformations, while the conformational changes of the syn-type amphiphiles were largely restricted. Furthermore, the anti-type amphiphile having the highest structural flexibility showed a characteristic split Cotton effect in an organic medium and formed the largest aggregates upon addition of water with a significant change in the circular dichroism (CD) profile, while amphiphiles having conformational restriction by the syn-configuration or a macrocyclic structure showed monomodal CD signals and afforded significantly smaller aggregates upon addition of water. Hence, the C2-symmetric alkaloidal BPI structure is demonstrated to be a useful core scaffold for supramolecular chemistry to design amphiphiles with controllable configurational diversity, which allows for the customization of chiroptical properties, conformational flexibility and self-assembly.
- This article is part of the themed collection: Supramolecular chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...