Visible light catalyzed synthesis of quinolines from (aza)-Morita–Baylis–Hillman adducts†
Abstract
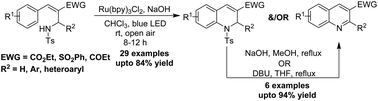
A mild and efficient protocol for the synthesis of quinoline scaffolds from (aza)-MBH adducts under visible light catalysis has been established. The reaction involves visible light catalyzed generation of amidyl radicals from (aza)-MBH adducts followed by intramolecular radical cyclization. The reaction exhibits a wide substrate scope, good functional group tolerance and high regioselectivity. This is the first example of utilizing (aza)-MBH adducts for the generation of amidyl radicals and synthesizing aza-heterocycles under visible light photoredox catalyzed reaction conditions.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...