Nickel-catalyzed regioselective arylation of aromatic amides with aryl iodides enabled by an N,O-bidentate directing group†
Abstract
A bidentate directing group enabled regioselective arylation of C(sp2)–H bonds in aromatic carboxamides with aryl iodides under nickel-catalysis is reported, which provides the corresponding products in moderate to good yields. This protocol using the inexpensive and low-toxic Ni catalyst can tolerate a wide range of functional groups.
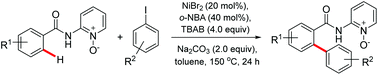
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...