I2-Triggered N–O cleavage of ketoxime acetates for the synthesis of 3-(4-pyridyl)indoles†
Abstract
A facile and complementary [3 + 2 + 1] annulation of aryl ketoxime acetates and 3-formylindoles to give pyridine derivatives is reported. The condensation reaction demonstrated that I2 was capable of triggering N–O bond cleavage of ketoxime acetates to generate iminyl radicals via a single electron transfer pathway. This direct and operationally simple protocol provides a fundamental platform to synthesize 3-(4-pyridyl)indoles with high functional group compatibility and high regioselectivity.
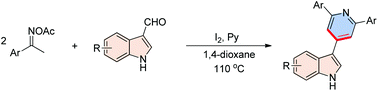
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...