Synthesis and mass spectrometric analysis of disaccharides from methanolysis of heparan sulfate†
Abstract
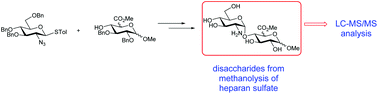
The quantification of heparan sulfate (HS) in biological matrices, e.g., urine, cerebrospinal fluid, tissue samples etc., is of great importance for the diagnosis and prognosis of several of the mucopolysaccharidosis (MPS) disorders, which are lysosomal storage diseases of impaired glycosaminoglycan metabolism. The development of suitable assays for this purpose is challenging due to the high molecular weight and complexity of HS. Recent efforts towards this goal include the acid catalysed methanolysis of HS, which desulfates the polymer and results in the formation of disaccharide cleavage products which can be detected and quantified by LC-MS/MS. We have synthesized a library of 12 HS-derived disaccharides as methanolysis standards via the stereoselective 1,2-cis glycosylation of suitably protected GlcA and IdoA acceptors with a 2-deoxy-2-azido thioglucoside donor. This facilitated identification of the major peaks in the LC-MS/MS chromatograms, and potentially will allow the monitoring of specific metabolites as surrogate markers for genotype. This work also paves the way towards a fully quantitative LC-MS/MS assay for HS via the preparation of a suitably labelled derivative.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...