Enantioselective biocatalytic formal α-amination of hexanoic acid to l-norleucine†
Abstract
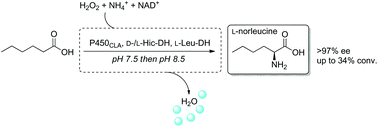
A three-step one-pot biocatalytic cascade was designed for the enantioselective formal α-amination of hexanoic acid to L-norleucine. Regioselective hydroxylation by P450CLA peroxygenase to 2-hydroxyhexanoic acid was followed by oxidation to the ketoacid by two stereocomplementary dehydrogenases. Combination with final stereoselective reductive amination by amino acid dehydrogenase furnished L-norleucine in >97% ee.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...