Mannich-type addition of 1,3-dicarbonyl compounds to chiral tert-butanesulfinyltrifluoroacetaldimines. Mechanistic aspects and chiroptical studies†
Abstract
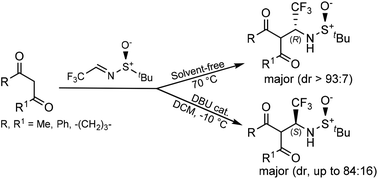
Mannich-type addition of 1,3-dicarbonyl compounds to (SS)-N-tert-butanesulfinyltrifluoroacetaldimine has been carried out under dramatically different conditions. The stereochemical outcome was quite different when the reaction was carried out under solvent-free conditions, at high temperature and without any catalyst or additive, compared with the DBU catalyzed reaction in dichloromethane solution at low temperature. Mechanistic aspects of the reaction under both the conditions are discussed. In this respect, vibrational (VCD) and electronic circular dichroism (ECD) and optical rotatory dispersion (ORD) experiments proved to be valuable tools for determining the absolute configurations of the reaction products.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...