First enantioselective total synthesis of altersolanol A†
Abstract
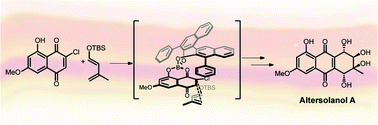
The first enantioselective total synthesis of altersolanol A, a secondary metabolite from the endophytic fungi Stemphylium globuliferum and Alternaria solani, is described. The key step towards the tetrahydroanthraquinone core was an asymmetric Diels–Alder (D–A) cycloaddition promoted by (R)-3,3′-diphenyl-BINOL/boron Lewis acid with good to excellent yields and excellent diastereo- and enantioselectivity (>95 : 5 dr and 98 : 2 er).
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...