Umpolung cyclization reaction of N-cinnamoylthioureas in the presence of DBU†
Abstract
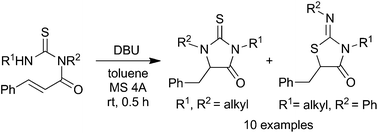
A novel regioselective cyclization reaction of N-cinnamoylthioureas leading to six- or five-membered heterocyclic compounds was developed. N-Cinnamoylthioureas in the presence of trifluoroacetic acid (TFA) underwent the well-established intramolecular cycloaddition reaction to give 2-imino-2,3,5,6-tetrahydro-4H-1,3-thiazin-4-ones in good yields. On the other hand, the reaction with 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) proceeded in an unprecedented “umpolung” cyclization fashion to afford five-membered 2-imino-1,3-thiazolidin-4-ones and/or 2-thioxoimidazolidine-4-ones. The reaction was considered to occur via a cycloadduct of DBU with the cinnamoyl moiety followed by intramolecular attack of the thiourea group.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...