Convergent total synthesis of (±) myricanol, a cyclic natural diarylheptanoid†
Abstract
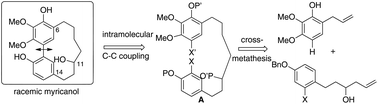
Myricanol 1, a constituent of Myrica species, has been reported to lower the levels of the microtubule-associated protein tau (MAPT), whose accumulation plays an important role in some neurodegenerative diseases, such as Alzheimer's disease (AD). Herein we described a new synthetic route to prepare myricanol in 9 steps and 4.9% overall yield starting from commercially available 2,3-dimethoxyphenol and methyl 3-(4-benzyloxyphenyl)propanoate. The key steps are a cross-metathesis to obtain a linear diarylheptanoid intermediate and a Suzuki–Miyaura domino reaction to generate the challenging macrocycle.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...