Structure-based protein engineering enables prenyl donor switching of a fungal aromatic prenyltransferase†
Abstract
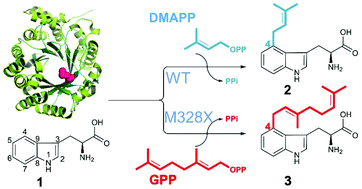
Microorganisms provide valuable enzyme machinery to assemble complex molecules. Fungal prenyltransferases (PTs) typically catalyse highly regiospecific prenylation reactions that are of significant pharmaceutical interest. While the majority of PTs accepts dimethylallyl diphosphate (DMAPP), very few such enzymes can use geranyl diphosphate (GPP) or farnesyl diphosphate (FPP) as donors. This catalytic gap prohibits the wide application of PTs for structural diversification. Structure-guided molecular modelling and site-directed mutagenesis of FgaPT2 from Aspergillus fumigatus led to the identification of the gatekeeping residue Met328 responsible for the prenyl selectivity and sets the basis for creation of GPP- and FPP-accepting enzymes. Site-saturation mutagenesis of the gatekeeping residue at position 328 in FgaPT2 revealed that the size of this side chain is the determining factor for prenyl selectivity, while its hydrophobicity is crucial for allowing DMAPP and GPP to bind.
- This article is part of the themed collections: Chemical Biology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...