Ruthenium-catalyzed tandem annulation/arylation for the synthesis of unsymmetrical bis(heteroaryl)methanes†
Abstract
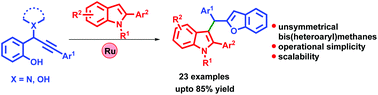
A new Ru-catalyzed tandem furan annulation/arylation strategy has been developed to afford unsymmetrical bis(heteroaryl)methanes by a reaction between propargyl amines and indoles. A series of bis(heteroaryl)methanes containing furan and indole as well as indole and imidazopyridine moieties have been synthesized in high yields. The reaction possibly proceeds through furan annulation followed by nucleophilic addition of indole.


 Please wait while we load your content...
Please wait while we load your content...