Palladium-catalyzed cascade carboesterification of norbornene with alkynes†
Abstract
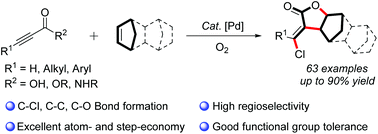
An efficient and convenient palladium-catalyzed cascade carboesterification of norbornenes (NBE) with alkynes has been accomplished to afford functionalized α-methylene γ-lactone and tetrahydrofuran derivatives in good to excellent yields. This new strategy exhibits excellent atom- and step-economy, good functional group tolerance and broad substrate scope. In particular, NBE-palladium species was proposed to be the key intermediate in the catalytic cycle to suppress the β-H elimination process. Notably, the developed protocol provides a straightforward and practical tool for the construction of diverse oxygen-containing heterocycle frameworks, illustrating a promising application in synthetic and pharmaceutical chemistry.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...