Different hybridized oxygen atoms controlled chemoselective formation of oxocarbenium ions: synthesis of chiral heterocyclic compounds†
Abstract
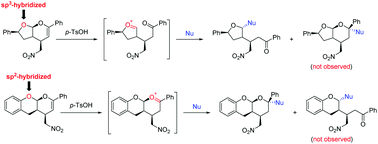
Based on the electronic properties of different hybridized oxygen atoms (sp3versus sp2) in the structure of O,O-acetals containing an enol ether moiety, the chemoselective formation of oxocarbenium ions was realized to furnish diversified chiral heterocyclic compounds with excellent stereoselectivities by reacting with different types of nucleophiles. Additionally, an unexpected intramolecular oxocarbenium ion transfer was also reported to form polycyclic products containing the O,O-acetal functional group.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...