Toward the total synthesis of patellazole B: synthesis of an advanced C1–C25 fragment corresponding to the macrocyclic skeleton†
Abstract
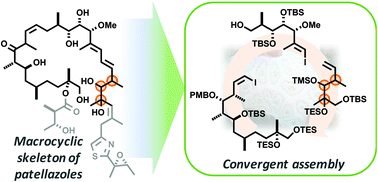
The patellazoles are a family of complex marine macrolides that exhibit potent cytotoxicity against cancer cell lines. However, despite extensive characterisation efforts, their full stereochemical assignment has remained elusive. We report our approach towards the synthesis-enabled structural elucidation of patellazole B (4), a 24-membered macrolide with 16 stereocentres and a signature thiazole-containing side chain. Our plan hinges upon isolating the unknown stereocentres into a single C20–C25 fragment to facilitate the flexible assembly of various possible diastereomers of an advanced C1–C25 fragment. Towards this end, a highly convergent and modular synthesis of one candidate diastereomer 37, corresponding to the patellazole B macrocyclic skeleton, has been achieved based on the strategic application of stereocontrolled aldol methodology, combined with Suzuki and Heck cross-coupling reactions.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...