Dioxane-involving reaction for the synthesis of 3-aryl-1-(2-(vinyloxy)ethoxy)isoquinolines catalyzed by AgOTf†
Abstract
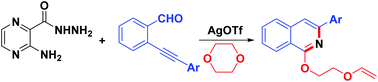
Dioxane was found to be involved in the reaction of 2-(arylethynyl)benzaldehydes and 3-aminopyrazine-2-carbohydrazide, and underwent a ring-opening reaction catalyzed by AgOTf. This domino type procedure provided an efficient method for the synthesis of 3-aryl-1-(2-(vinyloxy)ethoxy)isoquinolines in good yields via the loss of a molecule of 3-aminopyrazine-2-carboxamide.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...