Regioselective addition of phosphites to acyl cyclopropanes and following rearrangements: a facile access to enol phosphates†
Abstract
An inorganic base-promoted domino reaction with organophosphites and acyl cyclopropane derivatives is developed and proved to provide an efficient access to functionalized enol phosphates. Unlike the well-known Perkow reaction, which employs trialkyl phosphite as the nucleophile, dialkyl phosphite is the key to the success of our transformation. This method is compatible with a series of dialkyl phosphites and acyl cyclopropanes possessing electron-withdrawing substituents, and an array of functionalized enol phosphates are successfully prepared. Based on the results of isotope-labeling and control experiments, this transformation is presumably initiated by the deprotonation of dialkyl phosphite and the following nucleophilic addition/anion shift/ring opening sequence leads to the formation of enol phosphates. Both the strain release of a three-membered ring and the formation of a relatively stable anion are indispensable driving forces for this process.
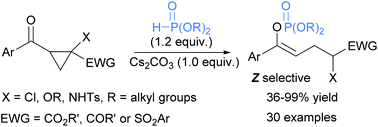
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...