Target-protein-selective inactivation and labelling using an oxidative catalyst
Abstract
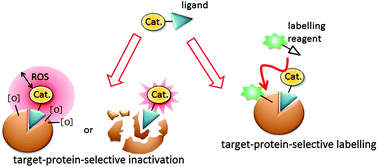
Reactive oxygen species (ROS) and radical species generated using oxidative single-electron transfer (SET) catalysts are highly reactive, inducing local environmental oxidative reactions, resulting in protein inactivation and labelling in proximity to the catalysts. Oxidative catalysts bound to the target protein generate ROS which induce oxidation only within a limited radius (∼30 nm), resulting in target-protein-selective inactivation. On the other hand, protein chemical labelling reactions via ROS or SET induced by the catalysts are completed in proximity to the catalyst. These proximity labelling techniques have recently attracted considerable attention as innovative tools to elucidate protein interaction mapping and unknown protein–protein interaction (PPI) partners. Not only can peroxidases be genetically introduced into the protein of interest but also ligand-conjugated catalysts can catalyze oxidative SET reactions in a protein mixture under intracellular conditions. In this review, we focus on two approaches of selective inactivation of protein functions and selective protein labelling using oxidative SET catalysts.


 Please wait while we load your content...
Please wait while we load your content...