Formation of Nα-terminal 2-dialkyl amino oxazoles from guanidinated derivatives under mild conditions†
Abstract
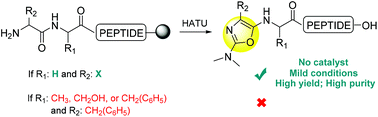
Oxazole-containing peptides are an important class of molecules in medicinal chemistry programs. Here we describe a convenient solid-phase synthesis of Nα-terminal oxazole peptides. The strategy took advantage of an intramolecular rearrangement side reaction that occurred during the guanidination of the Nα-amino function of a peptide still anchored on the solid-support. The substitution map of the N,N-dialkylamino oxazole obtained using this strategy differed completely from the one achieved through the heterocyclization of the Ser or Thr side chain with the preceding carbonyl group, which is a common approach for the preparation of these compounds. This unexpected reaction was observed with N-terminal aromatic and aliphatic amino acids that have a Gly as the last before residue in both short as well as long peptides; however, it does not form the oxazole ring if Gly was substituted with other amino acids.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...