Nickel-catalysed radical tandem cyclisation/arylation: practical synthesis of 4-benzyl-3,3-difluoro-γ-lactams†
Abstract
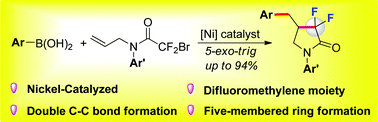
Enabled by nickel catalysis, a practical access to the synthesis of 4-benzyl-3,3-difluoro-γ-lactams has been developed via radical tandem cyclisation/arylation. This method features a nickel catalyst, high reaction efficiency, and good substrate tolerance and scope. This protocol proceeds through an intramolecular radical addition to form a primary alkyl radical followed by intermolecular Suzuki-type coupling.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...