Synthesis of highly substituted 2-spiropiperidines†
Abstract
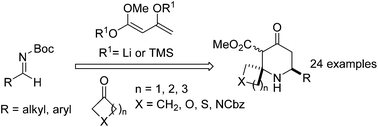
2-Spiropiperidines are a highly desirable, yet under represented structure in drug discovery. 2-Spiropiperidines were synthesised in either a two-pot or one-pot reaction. In the two-pot reaction, the addition of a Weiler dianion to N-Boc imines, followed by deprotection and in situ condensation with a cyclic ketone generated functionalised 2-spiropiperidines in good to excellent yields. In the one-pot reaction, the addition of Chan's diene to N-Boc imines under Maitland–Japp conditions, followed by the addition of sodium bicarbonate and a cyclic ketone formed functionalised 2-spiropiperidines in moderate to good yields.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...