Asymmetric organocatalytic synthesis of chiral 3,3-disubstituted oxindoles via a 1,6-conjugate addition reaction†
Abstract
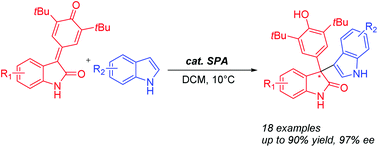
A highly efficient synthesis of chiral 3,3-disubstituted oxindoles was developed using a chiral spirocyclic phosphoric acid catalyzed 1,6-conjugate addition reaction of para-quinone methides derived from N-unprotected isatins with indoles. The reaction proceeds under mild reaction conditions to provide indole-containing N-unprotected oxindoles bearing quaternary stereocenters in good yields and with moderate to excellent enantioselectivities (up to 97% ee).
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...