Total synthesis and structural revision of an isopanepoxydone analog isolated from Lentinus strigellus†
Abstract
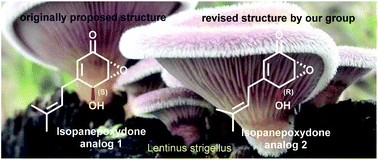
Asymmetric total synthesis of compound 1, as a proposed molecular structure of a natural product, in 11 steps is described. The inconsistency of the characterization data between our synthesized sample and the natural product prompted us to propose a different molecular structure as compound 2 and accordingly accomplish total synthesis in 9 steps and confirm the structural revision of this natural product. Both total syntheses feature highly regio- and diastereoselective epoxidation, Stille cross-coupling and cross-metathesis.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...